
This is the tendency of some minerals to appear in certain geometric shapes, which correspond to simple or combined crystallographic shapes.
The habit of minerals is the result of the internal structure of the crystals.
Habit can be seen macroscopically or with the help of a stereographic microscope, as in this figure, or under the microscope, as in the next screen.

By habit, in a microscopic preparation (thin section) mineral grains can be classified into three types: euhedral, subhedral and anhedral.
![]() The euhedrals (or idiomorphs or automorphs) are those that tend to appear under a certain geometric shape. Its geometric faces are well defined.
The euhedrals (or idiomorphs or automorphs) are those that tend to appear under a certain geometric shape. Its geometric faces are well defined.
![]() In subhedrics (or subidiomorphs or hipidiomorphs) not all faces are well defined.
In subhedrics (or subidiomorphs or hipidiomorphs) not all faces are well defined.
![]() Finally, the anhedral (allotriomorphs, xenomorphs) do not have defined shapes, all their faces are irregular.
Finally, the anhedral (allotriomorphs, xenomorphs) do not have defined shapes, all their faces are irregular.
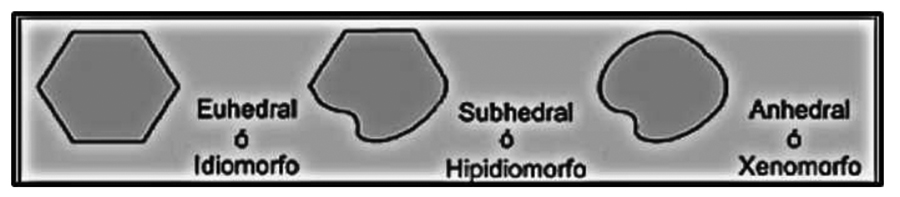
By the shape the crystals are defined as:
![]() Equidimensionals (octagonals). When no dimension predominates (frequently it is about minerals that crystallize in the cubic system).
Equidimensionals (octagonals). When no dimension predominates (frequently it is about minerals that crystallize in the cubic system).
With a shorter dimension (C axis, vertical axis) than the other two B and C axes, horizontal axes).
![]() Tabulars. They have a shorter dimension than the other two. They are in the form of a table.
Tabulars. They have a shorter dimension than the other two. They are in the form of a table.
![]() Laminars. When the shortest dimension has a minimum size. They are like overlapping sheets.
Laminars. When the shortest dimension has a minimum size. They are like overlapping sheets.
With a longer dimension (C axis, vertical axis) than the other two B and C axes, horizontal axes).
![]() Prismatics. One dimension longer than the other two. Prism shape.
Prismatics. One dimension longer than the other two. Prism shape.
![]() Ribbons/Aciculars. Prismatic crystals with a very high length/width ratio. Needle-like.
Ribbons/Aciculars. Prismatic crystals with a very high length/width ratio. Needle-like.
![]() Fibrous. As needle-like but with a value for the extreme length/width ratio. They are like fibers or hairs.
Fibrous. As needle-like but with a value for the extreme length/width ratio. They are like fibers or hairs.

These shapes are difficult to recognize under the microscope when working with thin slices that represent cuts of the samples, since the orientation of the cut will have a decisive influence on the shape of the section obtained. Minerals in microscopic preparations (two dimensions) sometimes show different shapes than they actually do macroscopically (in three dimensions).
Some examples of minerals are presented in the following figures.
 |
 |
 |
 |
 |
Index | Introduction | PPL | Previous | Next | Top