We refer in this program exclusively to the results of
reduction/oxidation of Fe and Mn taking place in the soil. Other processes
of hydromorphism, such as ferrolysis and clay illuviation in reducing conditions
will be treated in other programs.
What is hydromorphism?
Hydromorphism is considered as a permanent or temporary state of water saturation in the soil associated with conditions of reduction.
Hydromorphism has considerable effects on the soil, which are reflected as well in its composition, properties, genesis and evolution, as in its management properties (agriculture, engineering).
Its importance has been fully recognized and numerous terms have been created in different system of soil description and soil classification to indicate this state of the soil. In specialized literature following terms are frequently used: hydromorphic properties, mottled horizons, flecks with chroma lower than 2, decoloration, aquic moisture regimes, gleyification, pseudo- gleyification, redoximorphic features, etc..
Conditions required
![]() Saturation with water
Saturation with water
An vital requirement for the process of reduction is an excess of water in the soil during a given time.
In order to get such saturation with water it is first of all necessary to have a sufficient supply of it, and secondly its rapid elimination should be hindered (deficient drainage). The water supply may originate from a sufficiently superficial phreatic table, or from precipitation (rain or snow). In the latter case it is necessary that the supply of water is faster than its drainage, creating a temporary perched water table. This occurs mainly in soils with an abrupt textural change between the horizons, such as luvisols, where a more clayey Bt horizon is overlain by a more sandy E horizon.
![]() Absence of oxygen
Absence of oxygen
This condition is created easily in the soil each time the water stagnates in it and is not renewed. This is for instance the case in clayey soils with a slow internal drainage.
In these conditions the microorganisms consume quickly all oxygen dissolved in the water.
Sometimes the soil is subject to frequent supplies of superficial water (run-off or fluvial) which moves through it, and by remaining more oxygenated, do not cause reduction in spite of the high soil moisture. This situation is very typical for fluvisols.
![]() Presence of dissolved organic matter
Presence of dissolved organic matter
By moving slowly through the soil, water will be charged with organic residues and acquire a strong reducing character.
Soils which are very poor in organic matter do, in general,
not present hydromorphic features, unless they remain saturated with water
during a considerable time.
![]() High temperature
High temperature
Temperature should be sufficiently high in order not to limit biological activity. Due to the fact that the oxidation-reduction reactions are slow, the activity of microorganisms, which acts as catalysts, is in practice necessary. Therefore temperatures should remain above 5°C during the hydromorphic phase, as this is the limit generally accepted for microbial activity.
![]() A not too low pH
A not too low pH
As the reduction of Fe and Mn is a fundamentally biochemical
process, also pH will be a limiting factor.
Oxidation-reduction processes
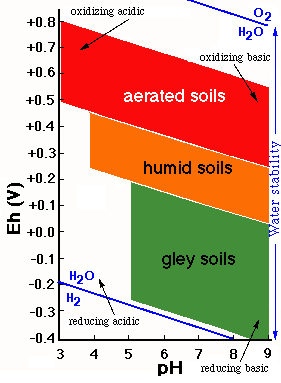
The behavior of soil materials affected by oxidation-reduction processes can be represented on a diagram on which, on the basis of the Eh and pH values, different fields of stability are delimited.
The aerated environments are oxidizing and correspond to high Eh values, whereas the environments saturated wich water are reducing and characterized by low Eh values.
The figure represents in a general way the usual values of Eh and pH for soils with three different degrees of moisture (aerated, humid and flooded).
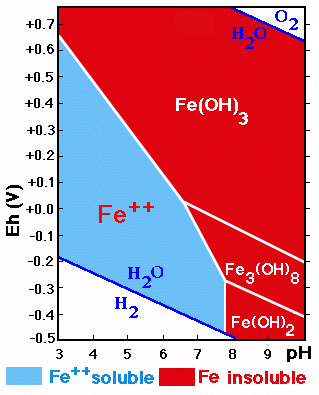
The behavior of the forms of Fe (and Mn) is strongly influenced by the redox conditions and the acidity/alkalinity existing in the soil.
The diagram shows the stability fields of the forms of Fe in function of the Eh/pH. The zone of Fe-precipitation is much larger than that of its solubility.
Iron reduced to Fe++ is soluble and corresponds to the pH region below 8 and low Eh-values.
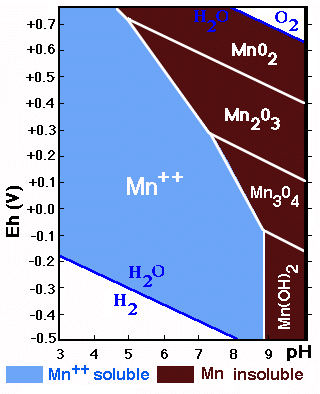
The diagram shows the stability fields of Mn-forms in function
of Eh/pH. The field of soluble Mn is larger than that of the precipitated
forms (contrary to what is happening with Fe).
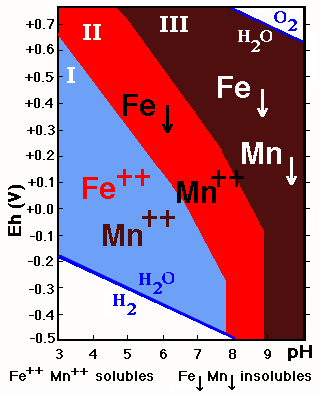
If the stability fields of Fe and Mn are superimposed three fields with different behavior are delimited:
![]() I. Mn++ and Fe++ soluble: acid soils
I. Mn++ and Fe++ soluble: acid soils
![]() II. Mn++ soluble and Fe precipitated: acid and
neutral soils with high Eh values, and slightly alkaline soils with low Eh values.
II. Mn++ soluble and Fe precipitated: acid and
neutral soils with high Eh values, and slightly alkaline soils with low Eh values.
![]() III. Mn and Fe insoluble: neutral and alkaline
soils with high Eh values and very alkaline soils.
III. Mn and Fe insoluble: neutral and alkaline
soils with high Eh values and very alkaline soils.
If one superposes the fields corresponding to aerated soils, humid soils and totally saturated ones, a series of interesting facts appears:
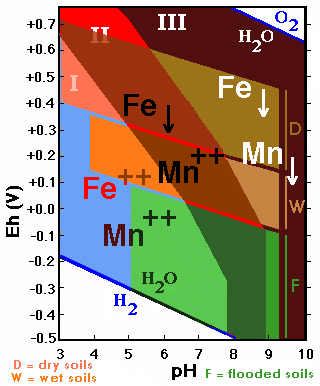
Aerated soils
![]() The iron is practically always insoluble (only
soluble in extremely acid soils with high Eh values);
The iron is practically always insoluble (only
soluble in extremely acid soils with high Eh values);
![]() The manganese is strongly influenced by the
pH: soluble in acid soils, insoluble in alkaline ones
The manganese is strongly influenced by the
pH: soluble in acid soils, insoluble in alkaline ones
Soils totally saturated with water
![]() The iron is in a soluble phase in acid soils,
and insoluble in alkaline ones;
The iron is in a soluble phase in acid soils,
and insoluble in alkaline ones;
![]() Manganese is practically always in a soluble
phase.
Manganese is practically always in a soluble
phase.
It is interesting to emphasize that manganese is always reduced at much higher Eh values than iron, and therefore is the first to be mobilized when the soil gets wet, and the last to oxidize (and thus become immobile) when it becomes dry. Mn is thus the most mobile, as shown in the figure, which represents the different possible variations of the Eh values of a soil subject to changes in humidity in different conditions: one of short hydromorphism (vertical blue line) and another with a much stronger hydromorphism (vertical yellow line).
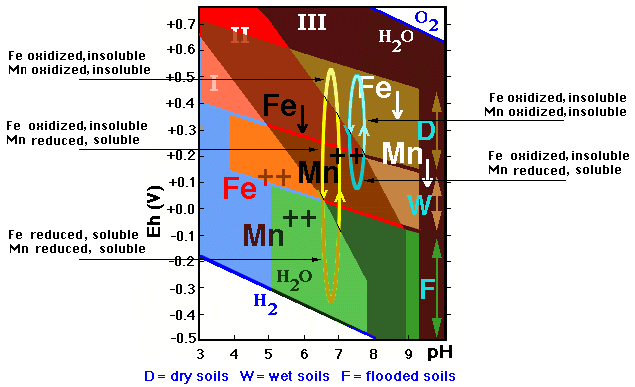
![]() In the first case the decrease of the Eh-value
is weakly expressed and only affects the Mn which becomes reduced, and therefore
mobile and, if not leached out the profile, will accumulate as nodules and (hypo)
coatings when the soil becomes dry again.
In the first case the decrease of the Eh-value
is weakly expressed and only affects the Mn which becomes reduced, and therefore
mobile and, if not leached out the profile, will accumulate as nodules and (hypo)
coatings when the soil becomes dry again.
![]() In the second case the Eh-drecrease is sufficiently
marked to affect also the iron.
In the second case the Eh-drecrease is sufficiently
marked to affect also the iron.
Under normal conditions, water saturation therefore corresponds to reduction, the manganese and iron occurring as soluble Fe++ and Mn++ ions, get redistributed throughout the profile forming reduced compounds (with grayish, more or less bluish or greenish colors) or are removed from the soil (producing bleaching), leaving finally more or less grayish horizons.
In dry conditions on the contrary an oxidizing environment prevails, Fe and Mn occur in an oxidized state and are therefore immobile, accumulating in the lower soil as compounds with intense black, red, brown or yellow colors.