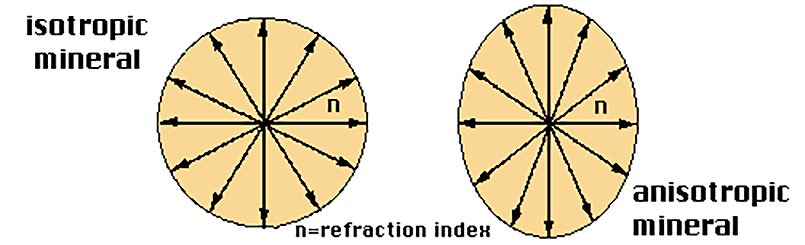
Isotropic and anisotropic minerals
This characteristic is presented by crystalline substances. Their behaviour depends on the direction in which the external agent is acting varies. In the case of light, it is translated into a change in the refractive index according to the direction of vibration of the light inside the mineral.
Supposing there were a luminous point in the centre of the mineral, the light would reach the outside of it at the same moment, creating a circumference for an isotropic mineral (equal velocity in any direction) and an ellipse in the case of an anisotropic mineral (different velocity according to direction).

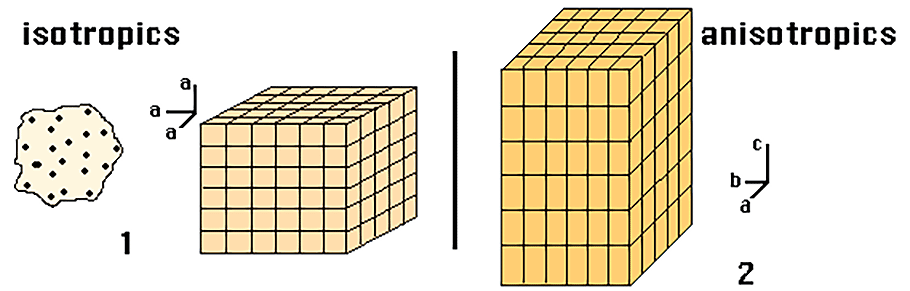
Anisotropy is related to the structure of the mineral, in that if there is no internal organisation (amorphous minerals) or the internal organisation is very regular, the minerals behave like isotropic minerals (1); otherwise they are anisotropic (2).
Amorphous minerals and those which crystallise in the Cubic System (also known as the Regular System) are Isotropic. The ions or atoms in isotropic minerals have an equivalent arrangement along all crystallographic axes.
Those which crystallise in the other systems are Anisotropic. The pattern of atoms varies with direction and thus the elasticity of the mineral also varies in relation to the vibration of the light waves.
<
Index | Introduction | Previous | Next