The translocation of carbonates is governed by the balancing reaction between carbonates (insoluble) and bicarbonates (soluble) in accordance with the following equation:
decarbonatation --------------------------------------------->
CaCO3 (insoluble) + CO2 + H2O <-----> Ca++ + 2HCO3-
<------------------------------------------------ carbonatation
The influential parameters are:
![]() Water
Water
The enormous importance of the water's concentration in the behaviour of the carbonates in the soil can be deduced from the equation. An increase in water will produce a displacement of the equilibrium towards the right with the consequent dissolution of carbonates, whilst on the other hand, a decrease in water will produce a corresponding precipitation and immobilisation of the carbonates.
more water => dissolution
less water => precipitation
![]() CO2
CO2
The enormous importance of the CO2 concentration in the behaviour of the carbonates in the soil can be deduced from the equation. An increase in CO2 will produce a displacement of the equilibrium towards the right with the consequent dissolution of carbonates, whilst on the other hand, a decrease in CO2 will produce a corresponding precipitation and immobilisation of the carbonates.
The action of CO2 on the dissolution of the carbonates is of great significance, as the partial pressure of CO2 in the soil air is in the order of 10 times greater than that of the atmosphere or even more. This accumulation of CO2 is due to biological activity (the action of roots and respiration of microorganisms) and the decomposition of organic matter.
increase in CO2 --> dissolution of carbonates
decrease in CO2 --> precipitation of carbonates
|
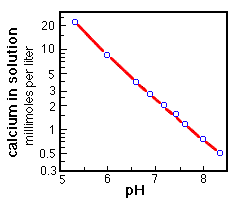
Changes in pH will affect the system, in that when the CO2 content of the water increases, the acidity will increase proportionately, and the dissolution of carbonates will occur. The opposite will happen if the alkalinity increases. increase in pH --> dissolution of carbonates decrease in pH --> precipitation of carbonates |
 |
|
|
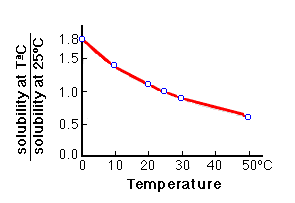
Temperature affects the equilibrium, as CaCO3 is less soluble in hot water than in cold water. The solubility of carbonates decreases with increasing temperature, and therefore the mobility of carbonates will be greater in cold climates than in warm ones. decrease in temperature --> dissolution de of carbonates increase in temperature --> precipitation of carbonates |
 |
![]() Salts
Salts
Another parameter which influences the solubility of the carbonates is the concentration of the soil solution: the effect that dissolved salts have on the solubility of a particular salt is well-known.
Thus, the presence of a common ion reduces the solubility, whereas the presence of other salts that do not have an ion in common with carbonates increases the solubility of the particular salt.
Index | Introduction | Previous | Next